Goal: Distinguishing components of the Lorentz force
Source: 283 – effects of magnetic force
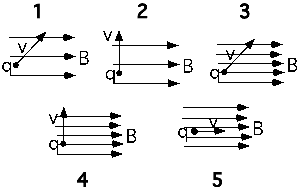
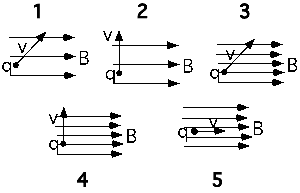
A charged particle moves into a region containing both an electric and
magnetic field. Which of the statements below are true?
- The particle cannot accelerate in the
direction of B.- The path of the particle must be a circle.
- Any change in the particle’s kinetic energy is caused by the E
field.
- Only A
- Only B
- Only C
- Both A&B
- Both A&C
- Both B&C
- All are true.
- None are true.
Commentary:
Answer
(3) The only cases that most students see is the one having E and B
perpendicular. As a result they discount the case of E and B parallel
and think statement A is also true.